
- EASY WATER THERMODYNAMICS CALCULATOR HOW TO
- EASY WATER THERMODYNAMICS CALCULATOR SERIES
Firstly, what is specific heat capacity, and why is a specific heat capacity calculator useful for mixtures? To answer these questions, let’s analyze the units of specific heat capacity. How Does the Rule of Mixtures Work?Īs previously stated, the calculator uses the rule of mixtures formula to estimate the specific heat capacity of a solution with multiple components. The mass of each individual component is equivalent to the total mass of the mixture, which can theoretically be broken down into separate parts.
Phase Change Materials Improve the Structural Integrity of Asphalt.How Does Salt Affect the Boiling Point of Water?.Does Adding Salt to Water Improve Boiling Time?.
EASY WATER THERMODYNAMICS CALCULATOR HOW TO
How to Use the Rule of Mixtures Calculator.Breaking Down the Rule of Mixtures Mixture?.The following post delves into the theory behind The Rule of Mixtures Calculator, how to use it, and present real-world examples to demonstrate its usefulness. In addition to the Rule of Mixtures Calculator, a materials database that includes the specific heat capacities of 1000+ materials can be found on the Thermtest website. Using the mass and specific heat capacity of each component, the Rule of Mixtures Calculator calculates the specific heat capacity of the entire sample. The Rule of Mixtures Calculator, newly released by Thermtest Inc., is an invaluable tool for estimating the specific heat capacity of mixtures containing any number of materials. If you are looking to use the calculator, click the button below. Note: This is a blog post describing the use of the Rule of Mixtures Calculator. Introducing the Rule Of Mixtures Calculator
EASY WATER THERMODYNAMICS CALCULATOR SERIES
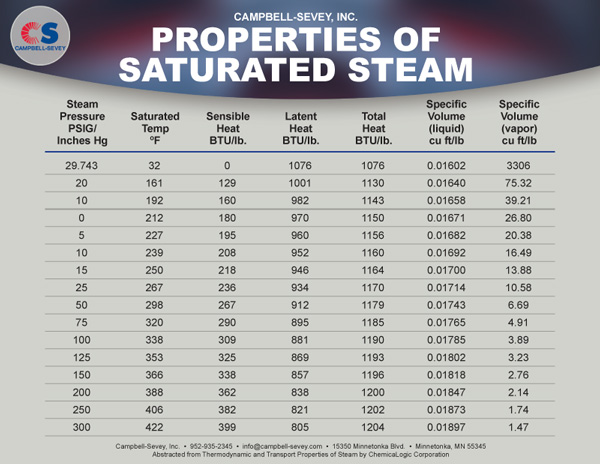
Thermal Resistance in Series Calculator. Thermal Conductivity & Thermal Resistance Calculator. TLS-100 Soil, Small to Medium Particle Size. MP-1 with THW Small to Medium Particle Size. MP-1 with TPS Small to Medium Particle Size. TPS-2 Viscous Liquids, Temperature & Pressure. MP-1 with THW Liquids, Temperature & Pressure. MP-2 Concrete, Rock, Polymers, and Insulation. HFM-25 Homogeneous & Heterogeneous Insulation. GHFM-01 Heterogeneous, Metals & Composites. HFM-50 Homogeneous & Heterogeneous Insulation. HFM-100 Homogeneous & Heterogeneous Insulation. TPS-2 Homogeneous & Heterogeneous, Insulation, Metals, Composites, Anisotropic & Thin-films. MP-1 with TPS Homogeneous & Heterogeneous, Insulation, Metals, Composites, Anisotropic & Thin-films. Transient Line Source Thermal Conductivity & Thermal Resistivity. Guarded Heat Flow Meter Thermal Conductivity & Thermal Resistance. Heat Flow Meter Thermal Conductivity & Thermal Resistance. Transient Hot Wire Thermal Conductivity, Thermal Diffusivity & Specific Heat. Modified Transient Plane Source Thermal Conductivity, Thermal Diffusivity, Specific Heat and Thermal Effusivity. Transient Plane Source Thermal Conductivity, Thermal Diffusivity and Specific Heat. This process takes a tremendous amount of energy, and that energy accounts for the large amount of energy it takes to boil water to make steam in electrical generating plants of all kinds (including nuclear), and for the efficient means humans have of cooling our bodies: perspiration. Notice that the largest contribution to this energy, by far, is in evaporating the water - changing it from liquid to gas. Here we use the heat of vaporization of water: Step 2: Convert the liquid water to steam at 100˚C. Below we'll do an example of a heat calculation as the temperature of a substance rises through a phase change. The Wikipedia page of a compound is usually a good place to find them. Compared to most other substances, it takes a large amount of heat to melt water ice and to boil or evaporate water.Įnthalpies of fusion and vaporization are tabulated and can be looked up. The relatively large attractive intermolecular forces between water molecules gives water very high heats of fusion and vaporization. Water has no more phase transitions after this. Finally, gaseous water above 100˚C absorbs heat, increasing its temperature at a constant rate. This is the latent heat of vaporization, ΔH v, the energy it takes for water to have no more cohesive force.Į. Water at 100˚C absorbs a great deal of heat energy at 100˚C as it undergoes a phase transition from liquid to gas. Heat is added to liquid water above 0˚C, and its temperature rises at a constant rate until the boiling point at 100˚C.ĭ. During the addition of the latent heat of fusion ( ΔH f), no temperature rise is observed, but hydrogen bonds holding the ice together break.Ĭ.






 0 kommentar(er)
0 kommentar(er)
